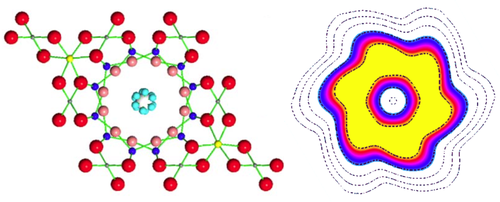
Water molecules confined in nanochannels exhibit tunneling behavior that smears out the positions of the hydrogen atoms into a pair of corrugated rings.
Tunneling is the “quantum super power” that lets particles go through microscopic barriers in a single bound. A study of water trapped in an emerald-like crystal reveals tunneling of water molecules among multiple orientations, so that each molecule is essentially in six configurations at once. The researchers showed with neutron scattering experiments that the tunneling causes the water’s hydrogen atoms to spread out into ring-like distributions. This new form of water is a more symmetric structure that is predicted to have zero electric dipole moment—the property that normally allows water to form hydrogen bonds and perform well as a solvent.
Tunneling occurs when an object traverses a barrier without having enough energy to do so classically. Certain molecules can tunnel among rotational orientations. A representative example is the methyl group (CH3)(CH3), which is a carbon atom bound to three hydrogens in a symmetric pyramid configuration. Electric forces from nearby atoms generate repulsion that resists any rotation around the pyramid axis. However, the hydrogens can tunnel through these barriers from one pyramid corner to the next. This discrete hopping couples together rotational orientations, causing an observable splitting of the ground state into multiple levels with slightly different energies.
Recently, optical spectroscopy revealed energy splitting in the terahertz spectrum of water molecules in the gemstone beryl, suggesting that the molecule is hopping among multiple states [1]. The crystal structure of beryl (Be3Al2Si6O18)(Be3Al2Si6O18) contains channels with hexagonal cross-sections that can trap water molecules. The channels periodically narrow into “cages” roughly 0.5 nanometers wide by 0.9 nanometers long and only big enough for one water molecule. The previously observed splitting suggested that the confined water was rotationally tunneling inside the channels, but a more direct test was necessary. Now Alexander Kolesnikov from Oak Ridge National Laboratory (ORNL) in Tennessee and his colleagues have performed a series of neutron scattering measurements on a beryl sample containing water.
In the team’s first set of experiments, they used low-energy (meV-scale) neutrons from the Spallation Neutron Source at ORNL to probe water-filled beryl. The team identified seven peaks in the scattering spectrum associated with water trapped in the beryl channels. As the temperature rose from 5 K to 50 K, these peaks decreased in height, implying that they come from molecular transitions related to quantum tunneling, rather than from vibrational transitions, which would have the opposite temperature dependence.
To explain their results, the researchers conducted first-principles calculations and found that a water molecule can occupy six symmetrical orientations in a beryl channel, in agreement with the known crystal structure. A single orientation has the oxygen atom roughly in the center of the channel, with the two hydrogens pointing to the same side (like a “<” symbol) toward one of the channel’s six hexagonal faces. Other orientations point to other faces, but are separated from each other by energy barriers of around 50 meV. However, these barriers don’t stop the hydrogens from tunneling among the six orientations and thereby splitting the ground-state energy into multiple levels. The energy differences among these levels were consistent with the seven peaks observed in the neutron scattering data, the researchers found.
The team performed a second set of experiments with high-energy neutrons from the ISIS neutron facility at Rutherford Appleton Laboratory in the UK. From these data, they found the hydrogens’ kinetic energy to be 30% lower in beryl than in water’s normal liquid or solid state. The lower energy implies that the hydrogens are less confined, as the tunneling frees them to be at the six positions simultaneously. The resulting charge density of each hydrogen is smeared out into a corrugated ring.
This smearing out, or “delocalization,” of the hydrogen atoms has a dramatic effect on the shape of the water molecule. Normally, water has an electric dipole moment resulting from its asymmetry, with the oxygen more negative and the hydrogens more positive. In beryl, the team predicts that this dipole disappears. Moreover, the center of mass would shift to the central axis of the double ring structure. Kolesnikov says this tunneling-induced shape-shifting may occur in other confined spaces where water is found, such as cell membranes or mineral interfaces.
The hydrogen atoms are spread into rings, so “the molecule can thus be considered as being in a new state,” says Boris Gorshunov of the Moscow Institute of Physics and Technology. He says that this configuration is consistent with his own group’s terahertz spectroscopy of beryl.
This research is published in Physical Review Letters.
–Michael Schirber
Source: Physics – Focus: Water Molecule Spreads Out When Caged












Leave A Reply