So-called “supercoils” change the behavior of DNA, opening a new role for topology in the study of life.
DNA is probably best known for its iconic shape — the double helix that James Watson and Francis Crick first described more than 60 years ago. But the molecule rarely takes that form in living cells. Instead, double-helix DNA is further wrapped into complex shapes that can play a profound role in how it interacts with other molecules. “DNA is way more active in its own regulation than we thought,” said Lynn Zechiedrich, a biophysicist at Baylor College of Medicine and one of the researchers leading the study of so-called supercoiled DNA. “It’s not a passive [molecule] waiting to be latched on to by proteins.”
The research hints at an unstudied language of DNA topology that could direct a host of cellular processes. “It’s intriguing that DNA behaves this way, that topology matters in living organisms,” said Craig Benham, a mathematical biologist at the University of California, Davis. “I think that was a surprise to many biologists.”
No Time to Relax
To get a sense for supercoiled DNA, imagine twisting a piece of string. Let the string go, and it unwinds. Twist it enough, and it folds back on itself. The degree of twist puts stress on the string, which governs the shape it takes.
DNA behaves in a similar fashion. Like the string, it prefers to be in its most relaxed state — the iconic double helix. But DNA rarely gets to relax. It’s subject to a continual onslaught of molecules that bind it — the enzymes that untangle, unwind and then replicate DNA; the molecules that mark which genes are active and which are silent; and the proteins that pack the lengthy molecule into a manageable size. All of these molecules contort DNA into new shapes, blocking it from the repose of the simple double helix.
Irobalieva RN et al., Nat. Comm. 2015
This simulation illustrates the dynamic dance of tiny circles of supercoiled DNA.
These interactions represent the inner workings of the cell, the basis of all life. How the cell decides to activate a certain gene, for example, involves a complex assembly of molecules in the right place at the right time. Protein-DNA interactions also present prime targets for drugs as well as insights into disease. Imagine a drug that could block activation of a cancer-linked gene without interfering with other genes.
Unfortunately, these interactions are very difficult to study because biological molecules morph shapes so easily. A mechanic would have a hard time fixing a car if the parts constantly mutated.
To capture the complex structure of these nanoscale interactions, scientists typically crystalize the molecules, freezing their shape for the camera. The vast majority of these studies use short strands of relaxed DNA — the standard double-helix form — because they’re easy to work with and cheap to make. But that may not capture the true picture; relaxed DNA often behaves differently than that found in the cell, contorted around all manner of proteins.
Zechiedrich and her collaborators have spent the last two decades making small pieces of supercoiled DNA, whose behavior better mimics DNA in the living cell. Essentially, they take a short strand of DNA and twist it — once, twice, three times or more — either with or against the coil. Then they glue the ends together. The end result is a tiny circle of DNA coiled in one direction or another. Zechiedrich, her collaborator and Baylor colleagueJonathan Fogg and others have shown that these twisted coils dance, shimmying through a microscopic ballet. Each molecule can assume a variety of shapes, from simple circles to figure eights, racquets, handcuffs, needles and rods. “Linear DNA is stiff and inflexible,” said De Witt Sumners, a mathematician at Florida State University in Tallahassee. “But when you get it bent into a small circle, the duplex opens up and adopts a large number of interesting shapes — this is completely unexpected.”

The newest study from Zechiedrich’s lab provides the clearest picture yet of these tiny rings. The researchers captured microscopic images of individual circlets in a variety of diverse shapes. Pairing the images with sophisticated computational models created by collaborator Sarah Harris, a biologist at the University of Leeds, they were able to predict the precise movements of each molecule.
Though scientists already knew bits and pieces of how supercoiled DNA functions, the combination of microscopy and modeling in the new paper helps to create a more precise picture. “For a large part of the biological community, seeing is believing,” said Stephen Levene, a biophysicist and bioengineer at the University of Texas, Dallas, who was not involved in the study. “You can show math models, but unless you have some convincing structural data, it’s hard to get people to appreciate what’s going on.”
DNA Exposed
Researchers have known since the 1970s that twisting DNA opposite the direction of the helix — called negative supercoiling — can split open the two strands. This split serves a dual purpose. It both relieves pent-up molecular stress and exposes the code hidden within the helix, granting access to the molecular machines that replicate DNA and make RNA.
But soon after that work was done, scientists developed new techniques to read the sequence of the base letters in the genome, launching the genetic sequencing revolution. “Sequencing opened up a lot of possibilities, but it also sidetracked everyone, so that [structural] questions were suddenly very passé,” said Benham.
For three decades, most scientists assumed that supercoiling probably wasn’t very important in complex cells, which have special enzymes that snip and untangle knotted DNA. These enzymes help prevent the buildup of troublesome stress. But they aren’t 100 percent effective. In 2008, Levens, the National Cancer Institute biologist, led a team that detected supercoils in human cells, reigniting interest in DNA’s higher-order structure.
Levens and collaborators found that transcription twists DNA, leaving a trail of undercoiled (or negatively supercoiled) DNA in its wake. Moreover, they discovered that the DNA sequence itself effects how the molecule responds to supercoiling. For example, the researchers identified a specific sequence of DNA that’s prone to opening when stressed, like a weak spot in an old inner tube. The segment acts as a sort of chemical cruise control; as the amount of supercoil rises and falls, it slows or speeds the pace at which molecular machinery reads DNA.
Levens says these structural changes also help DNA communicate along its length. Just as pressing an inner tube makes a weak spot bulge, changes in the shape of one part of the DNA molecule might trigger stress elsewhere along its length, which in turn might help regulate genes.

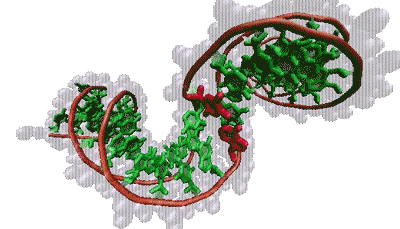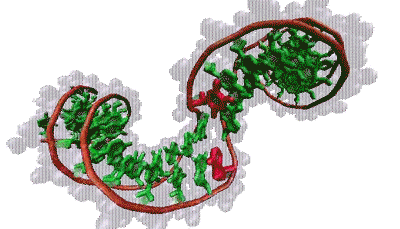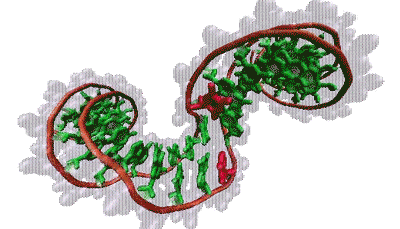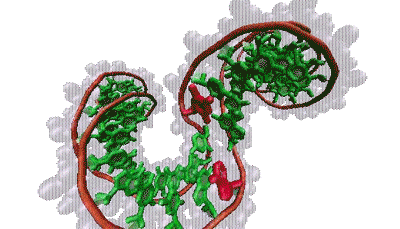
Thana Sutthibutpong and Sarah Harris
The molecular bases that make up our genetic code (green) are typically hidden within the double helix. But twisting the helix can turn certain bases (red) toward the outside.
The findings align with Harris’s models, which show that supercoiling can split the two strands of the helix, rotating the DNA bases that normally lie inside the helix to the outside, a phenomenon known as base flipping. Other simulations show that twisting a bit more flips out additional bases, creating a bubble of inside-out DNA. Zechiedrich theorizes these bubbles might provide trigger points for replication or gene expression. This challenges the standard view, in which proteins latch onto DNA and launch these events. “Who’s driving the bus in cellular metabolism?” said Sumners. “It’s a very dynamic process — DNA and proteins each influences how the other acts and reacts.”
Scientists hope the results will inspire new questions and a renewed consideration of DNA’s shape and flexibility. “These experiments are going to stimulate a lot of thinking and rethinking, especially in the physics community,” said Wilma Olson, a biophysical chemist at Rutgers University in New Jersey.
Mathematicians and physicists have long been intrigued by supercoiled DNA and the role that DNA topology plays in the cell. According to Sumners, the field is ripe for exploitation with new mathematical approaches. “Mother nature clearly has a message here,” Sumners said. “The question is how to interpret it.”
This article was reprinted on ScientificAmerican.com andBusinessInsider.com.
Source: DNA Supercoils Change the Way That Cells Work | Quanta Magazine












Leave A Reply